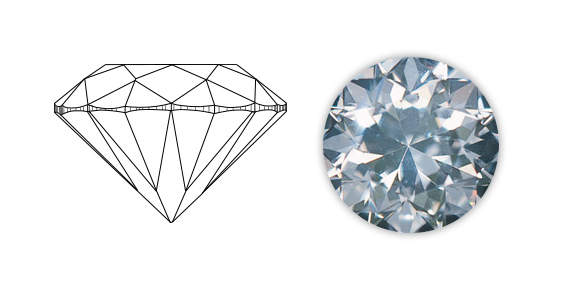
Why the diamond is so bright and shiny(brilliance)? Why it is so colorful (fire)? Let us explore.
The diamond is a form of carbon, lived inside the earth for thousands of years, hardened by the pressure; mined out; cut and polished; and sold by the slogan "diamonds are forever", and given the status as "the great gift to one's lady-love."
Let us go into some optical characteristics of a diamond. The diamond has a very high refractive index of 2.4 compared to water (1.3) and glass (1.5). That means, the light rays are bent (refracted) by a very large angle when they pass through a diamond. Consequently, it has a very less critical angle of 24 degrees. That means, a light ray which tries to emerge from the diamond at or more than 24 degrees to the vertical, will not pass out instead it will be reflected internally. Hence, mostly all the light rays undergo multiple reflections within a piece of diamond giving the characteristic brilliance.
A glass prism disperses (split up) white light into colors. The dispersion is more in the diamond. That is, colors are widely separated. That is why a well-cut diamond produces a remarkable changing color(fire) in bright light.
To facilitate 'brilliance and fire' the diamond should be cut in proper angles. In 1899, Marcel Tolkowsky from Antwerp analyzed the physics of diamond and gave a procedure to cut the diamond in his book "diamond design". He gave a recipe for "brilliant cut" with 58 facets. Hence the diamond is drawn in this "classic cut and shape" everywhere.
It is true that no other gems have this kind of an optical property.
Diamond is said to be the hardest material in the world. But, by gifting this hard diamond to someone, you may get a soft corner in the person's heart.
---------------------------------------