WHY CAN,T YOU UNSCRAMBLE AN EGG?
ds is greater than 0
“Energy should be greater than zero”.
Disorder should increase.
All above are different forms of second law of thermodynamics.
Think of Mercides Benz. Its heart is “internal combustion engine”. It works because of second law of thermodynamics.
In a cylinder, petrol and air is mixed ; pressed by piston; ignited. The mixture explodes, expands, and pushes the piston. The piston rotates the wheel through the lever and gear arrangement.
ROLE OF SECOND LAW IN BENZ
The gas mixture expands because the second law says that everything will go into disorder.
The explosion inside the cylinder is hot and the surrounding is cold. Hence the generated heat can pass outside; the next explosion can take place, and the engine can run.
An engine requires a reversible process. The reversible process can take place only when two bodies at different temperatures are available. (II law).
Heat can only flow from hot body to cold body. (From engine to surroundings) and not in the other direction.- again second law.
Hence automobile came into being because of second law of thermodynamics. Let us go into the heart of second law. It simply says “A process will take place only when disorder (entropy) increases”. There are many consequences for second law.
Ice melts into water. Because water is having more disorderly arrangement of molecules. Water cannot crystallize into ice spontaneously, because ice has orderly arrangement of molecules. A glass cup can fall and break into pieces. Obviously more disorder.
IF A BALOON IS PIERCED, AIR RUSHES OUT. WHEN AIR OCCUPIES MORE VOLUME, IT IS MORE DISORDER. THAT IS WHY, AIR FROM THE ROOM CANNOT GO INTO THE BALOON (SMALL SPACE-LESS DISORDER) AND FILL IT UP.
These are all spontaneous processes. Why the changes take place in the direction of more disorder? Because, there is more probability for disorder than orderly arrangement. When you shuffle a deck of cards, it will always go into disorderly arrangement, it will “almost never” will go into order like 1,2,3….K, Q, J. It is because there is a million chance for disorder but only one chance for correct order.
Hot means more disorder. In a hot gas, the molecules are highly in random motion. Cooled gas means slow motion and less disorder. Freezing means solidifying and regular arrangement of atoms. Hence, heat will always flow to increase disorder and temperature. Heat always flows from hot body to cold body to make it hot. Heat cannot flow the other way.
What happens while cooking. The stove supplies heat. It is called hot body. The vessel and the contents are heated by stove. The food is cooked. Some work has been done. The vessel is removed and put on the dining table. The heat from the vessel goes to environment. Here the environment can be taken as cold body. After cooling, the vessel is ready for the next cooking. Hence, for doing any work, two bodies at two different temperatures are required. If everything is at the same temperature, work cannot be done. Car’s engine works because its temperature is raised higher than the surroundings. Even our body is an engine, it works because the body is slightly at higher temperature than the environment.
When an engine runs, heating and cooling alternatively takes place. That is reversible process . But there is always wastage of heat energy. There is no perfect reversible process. Hence no engine has 100% efficiency.
When an engine runs for a long time, everything is heated up. They all reach same temperature. Further work cannot be done. That is why “ever running, never stopping, self driving” perpetual machine is not possible. That is why, in all the engines cooling systems are in-built. (Note the cooling system also require energy). Also note, perpetual machines are not considered for patents since they violate second law of thermodynamics.
Second law even tells that how the universe will end. The universe goes on expanding. Consequently, the temperature goes on increasing. Everything will reach the same high temperature -the ultimate disorder. Than, no work can be done. No life is possible. Finally, universe will reach the ‘heat-death”.
The law also gives the meaning for the concept of time. As the time progresses, the spontaneous actions will continuously take place. Consequently the disorder or entropy will go on increasing. Hence, we can say entropy has the time stamp and it is measure of time. Also, it implies that time arrow has only forward direction.
Also note, when salt solution crystalizes; when living molecules self-assemble. They seems to be spontaneous. But they take energy from environment and create disorder elsewhere.
Now we understand, why we cannot unscramble an egg. (May be possible in movies).
Hence, second law gives the meaning for time; predicts the end of the universe; explains why spontaneous actions takes place in a particular direction; implies the importance of probability and also helped to invent car engine.
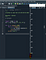
KEY IN ACTION-mini python program
This program selects the alphabets P,Q,R in random order and prints them-100 times.

A few sets of P Q R may be in order. But ,we mostly get disorderly Sets. Here, the last set has ordered elements.
when we randomly Select, P,Q,R and print them, we mostly get disordered arrangement. Random and spontaneous actions always result in disorder . Deliberate, energy consuming actions we'll result in order. This is the essence of "seconder law of thermo dynamics".